Like millions of others, I really enjoy listening to podcasts. One especially chilling and engaging podcast series was “Dr. Death” by Laura Beil. This same journalist has created a new series called Bad Batch.
https://wondery.com/shows/bad-batch/
This podcast details the true events that involves several patients (many in Texas) who sought out a “miracle cure” from “stem cells”. These patients were injected with umbilical cord blood-derived products produced by a company called Liveyon. After several patients became extremely sick, the samples were found to have been contaminated by bacteria. Through the ongoing series, we learn of the short-cuts that were taken that precipitated the adverse outcomes. This is a cautionary tale, but also a scary commentary on how greed and bravado can adversely skew good medical practices and taint the perception of practitioners who are trying to do things the right way.
Injections of biologic products should be viewed as a sophisticated medical procedure that is performed by licensed and trained physicians, or an Advanced Practice Provider (Nurse Practitioners, Physician Assistants, etc.) that practice under the direct guidance of a physician.
https://charmaustin.com/single-post/2019/09/19/The-CHARM-Difference
At CHARM, we use autologous (from YOUR BODY) biologic products including platelet-rich plasma (PRP) and bone marrow aspirate concentrate (BMAC). These products contain thousands of growth factors, proteins and mesenchymal stem cells that work together to help orchestrate tissue repair and decrease irritation in tissues that inherently have trouble healing. We do not use amniotic or umbilical products. We have made this decision for several reasons.
Amniotic and umbilical products:
– Have significantly higher cost because they are a third party product.
– Have dramatically lower volumes of solutions to use for treatment. So, we can’t treat structures as comprehensively.
– Present concerns of contaminants, preservatives, rejection (graft vs host response) or infectious agents. The latter is illustrated in the Liveyon case.
– Do NOT contain live, or viable, stem cells.
https://charmaustin.com/single-post/2019/06/14/Clearing-the-Air-About-Amniotic-Cells
– Have several questions about legality and FDA compliance. This is the boring and confusing part, but it is very important. The practitioners at CHARM have spent countless hours studying these regulations, and collaborating with physicians across the country, to ensure that our procedures are “above board” with the current regulatory environment.
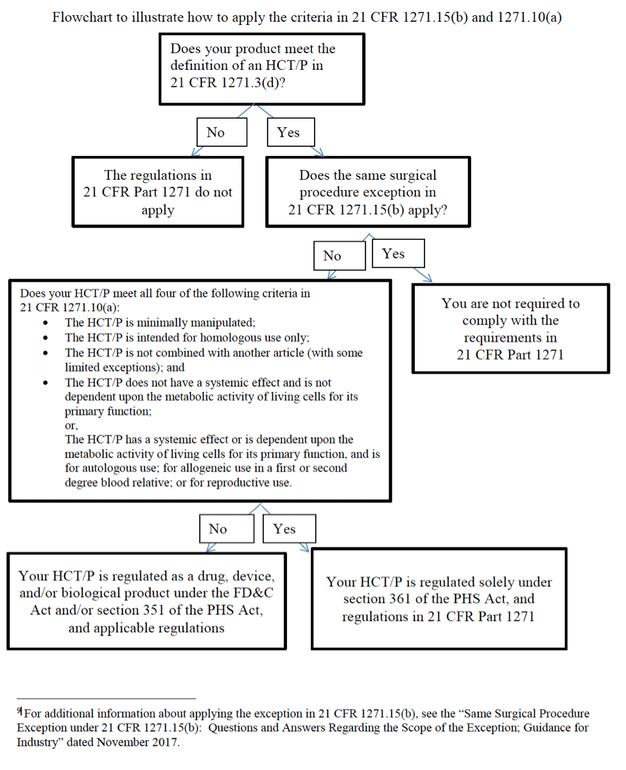
According to the FDA, the Center for Biologics Evaluation and Research (CBER) regulates:
In the U.S., human tissues intended for transplantation are regulated by the FDA as “Human cells, tissues and cellular and tissue-based products” or “HCT/Ps.”
HCT/Ps fall under two groups, commonly called “361” and “351” products. 361 products that meet all the criteria outlined in 21 CFR 1271.10(a) are regulated as HCT/Ps and are not required to be licensed or approved by the FDA. Bone marrow aspirate concentrate falls under this category when used for appropriate purposes.
In contrast, if a cell therapy product does not meet all the criteria outlined in 21 CFR 1271.10(a)), then it is regulated as a “drug, device, or biological product” under the Federal Food, Drug, and Cosmetic Act (FDCA) and Section 351 of the PHS Act. These 351 products (such as umbilical and amniotic products) used for orthopedic purposes need to be done in the setting of a medical study with an FDA approved Investigational New Drug (IND) application.
(See diagram below)
There are good and bad actors in medicine. We encourage our patients to ask questions and educate themselves on the risks, benefits and alternatives on whatever medical procedure is being presented to them.